Ethanol-blended fuels, while offering environmental benefits, present unique challenges when it comes to long-term storage. Unlike pure gasoline, ethanol is hygroscopic, meaning it readily absorbs water from the atmosphere. This characteristic is the primary driver of fuel degradation, leading to a host of problems including phase separation, corrosion, and reduced engine performance. Understanding the mechanisms of this degradation is crucial for implementing effective preventative measures.
The Challenge of Ethanol Fuel Degradation
The inherent hygroscopic nature of ethanol is at the heart of its storage issues. When ethanol-blended fuel, such as E10 (10% ethanol, 90% gasoline), comes into contact with moisture, it absorbs the water. Once a certain saturation point is reached, the water and ethanol mixture separates from the gasoline, sinking to the bottom of the fuel tank. This phenomenon is known as “phase separation.”
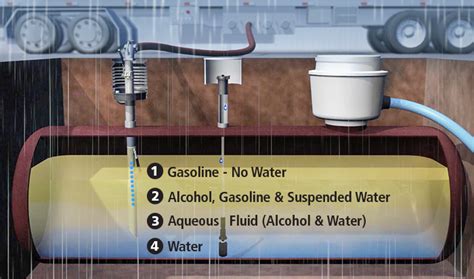
Phase separation is highly problematic because the separated, water-rich layer is corrosive to metal fuel tanks and components, particularly those made of steel, aluminum, and rubber. Furthermore, the remaining gasoline-rich layer has a lower octane rating, which can cause engine knocking and reduce efficiency. Over time, this degradation can lead to fuel system damage, clogged fuel filters, and significant operational issues for vehicles and small engines.
The Role of Fuel Stabilizers
To combat these issues, specialized fuel additives known as “fuel stabilizers” are essential for preventing ethanol fuel degradation during storage. These additives are formulated to address the specific challenges posed by ethanol, primarily by preventing water absorption and mitigating its harmful effects.

Key Components and Mechanisms of Action
Effective ethanol fuel stabilizers typically contain a blend of several active ingredients, each serving a specific purpose:
- Demulsifiers/Dispersants: These components work to either prevent water from bonding with ethanol or to help disperse small amounts of water evenly throughout the fuel, preventing it from settling and causing phase separation. Some formulations might also include agents that encapsulate water molecules, rendering them harmless.
- Corrosion Inhibitors: Since water and ethanol can corrode metal components, inhibitors form a protective layer on metallic surfaces, preventing rust and other forms of corrosion in fuel tanks, lines, and engine parts.
- Antioxidants: These agents prevent the oxidation of gasoline components, which can lead to gum and varnish deposits that clog carburetors and fuel injectors. While not directly related to ethanol’s hygroscopy, oxidation is another common form of fuel degradation in storage.
- Fuel System Cleaners: Some advanced stabilizers also include detergents or cleaners that help remove existing deposits and keep the fuel system clean, ensuring optimal performance when the fuel is eventually used.

It’s important to note that not all fuel stabilizers are created equal. Consumers should look for products specifically designed for ethanol-blended fuels, as generic gasoline stabilizers may not adequately address the unique challenges of ethanol.
Best Practices for Ethanol Fuel Storage
While a good fuel stabilizer is critical, it works best in conjunction with proper storage practices:
- Use a Sealed Container: Always store ethanol fuel in an approved, airtight container to minimize exposure to atmospheric moisture.
- Store in a Cool, Dry Place: Keep fuel containers away from direct sunlight and extreme temperatures, which can accelerate degradation.
- Add Stabilizer Proactively: Add the recommended amount of fuel stabilizer to the tank or storage container before storing the fuel, especially for seasonal equipment like lawnmowers, boats, or motorcycles. Run the engine for a few minutes after adding the stabilizer to ensure it circulates throughout the entire fuel system.
- Avoid Over-Storage: Even with stabilizers, it’s best to avoid storing ethanol-blended fuels for excessively long periods. Manufacturers often provide guidelines for stabilizer effectiveness (e.g., 12-24 months).

Conclusion
The prevention of ethanol fuel degradation in storage hinges on understanding its primary vulnerability: water absorption leading to phase separation. The most effective preventative measure is the use of a high-quality fuel stabilizer specifically formulated for ethanol-blended fuels. By combining these specialized additives with sound storage practices, vehicle owners and equipment operators can protect their fuel systems from damage, maintain fuel quality, and ensure reliable performance, even after extended periods of inactivity.